element with least electron affinity|4.4 Ionization energy and Electron Affinity – Chemistry : Clark We define the first electron affinity as the energy released when 1 mole of gaseous electrons are added to 1 mole of the element to form 1 mole of gaseous negative ions. First electron affinities are .
15 GP. PS4 版《NBA 2K14》實機遊玩簡短心得(介面介紹、MyCareer、公園模式) 作者:海芋鬱鬱│2013-12-26 20:55:34│巴幣:30│人氣:10143We have 3 different Uptown Aces no deposit promo codes totaling $30 in free chips plus 10 free spins! You can redeem all our no deposit bonus codes as long as you make a deposit in between .
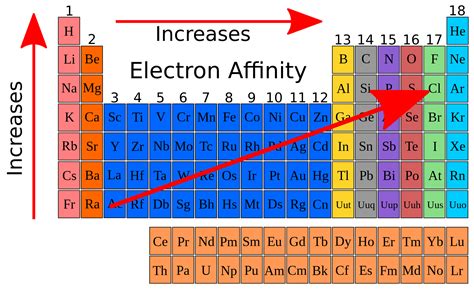
element with least electron affinity,Ago 11, 2023
4.4 Ionization energy and Electron Affinity – Chemistry The electronic affinity is amount of energy, that is released during the .
element with least electron affinity 4.4 Ionization energy and Electron Affinity – Chemistry The electronic affinity is amount of energy, that is released during the . Atoms with a low electron affinity want to give up their valence electrons because they are further from the nucleus; as a result, they do not have a strong pull on .
The electron affinity (EA) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. In general, elements with the .The element which has the least electron affinity is: A. Oxygen. B. Lithium. Carbon. D. Fluorine. Solution. The correct option is A. Oxygen. The explanation for the correct . We define the first electron affinity as the energy released when 1 mole of gaseous electrons are added to 1 mole of the element to form 1 mole of gaseous negative ions. First electron affinities are .
element with least electron affinityThe electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. So the more negative the electron affinity the more favourable the electron .

The sign of electron affinity (E EA) is opposite to the sign of energy change (ΔE). EEA = – (ΔE) So, if ΔE is positive, then E EA will be negative and if ΔE is negative, .

The sign of electron affinity (E EA) is opposite to the sign of energy change (ΔE). EEA = – (ΔE) So, if ΔE is positive, then E EA will be negative and if ΔE is negative, .
element with least electron affinity|4.4 Ionization energy and Electron Affinity – Chemistry
PH0 · electron affinity
PH1 · What is Electron Affinity?
PH2 · The element which has the least electron affinity is:
PH3 · Electron Affinity of The Elements
PH4 · Electron Affinity Chart of All Elements (With Periodic Table)
PH5 · Electron Affinity Chart (Labeled Periodic table + List)
PH6 · Electron Affinity
PH7 · CALCULLA
PH8 · 7.5: Electron Affinities
PH9 · 4.4 Ionization energy and Electron Affinity – Chemistry